Abstract
Introduction
So far graft-versus-host disease (GvHD) is the most common long-term complication after allogeneic stem cell transplantation (HCT) and it decreases the success of HCT by increasing the risk of death and disability. Chronic GvHD (cGvHD) is the leading cause of non relapse mortality in HCT survivors.
In 2011 the CIBMTR proposed a cGvHD risk score based on 10 variables - age, prior acute GvHD, time from HCT to cGvHD, donor type, disease status at HCT, GvHD prophylaxis, gender mismatch, serum bilirubin, Karnofsky score and platelet count - that provided stratification of patients (pts) into 6 risk groups (RG) with different outcome in terms of overall survival (OS) and non-relapse mortality (NRM). Recently the CIBMTR risk score was refined to enhance prognostic stratification with the incorporation of the absolute lymphocyte and eosinophil count at time of cGvHD onset.
However, few recipients of haploidentical HCT (haplo-HCT) were originally included in the CIBMTR risk score cohorts.
Methods
We analyzed the original and revised CIBMTR risk score in haplo-HCT at our Center, where a sirolimus-based, calcineurin inhibitor-free, GvHD prophylaxis was employed since 2006, to allow the safe infusion of unmanipulated haplo-HCT.
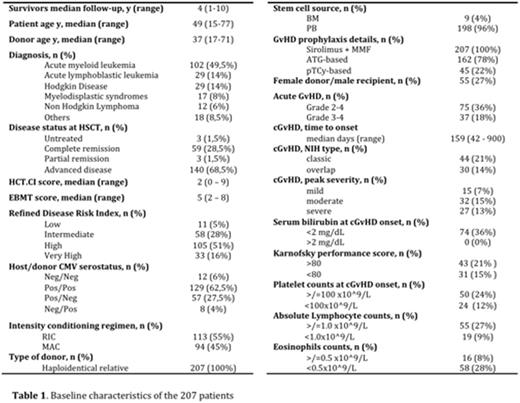
We analyze data consecutively collected between 2006 and 2014 including 207 adult pts. Data were prospectively collected in an Institutional database. A written consent was given by pts allowing the use of medical records for research in accordance with the Declaration of Helsinki.
All consecutive pts receiving a haplo-HCT as 1st allogeneic transplantation were included - pts receiving haplo-HCT as 2nd or 3rd HCT were excluded from the present analysis. All pts were prospectively evaluated for cGvHD according to the 2004 NIH consensus criteria.
Results
Baseline characteristics of the 207 pts are outlined in table 1.
With 4-year median follow-up, 4-year probabilities of transplant related mortality (TRM), relapse, progression-free survival (PFS), and overall survival (OS) were 25,8%, 42,5%, 31,7%, and 34,4%, respectively. Considering the composite end point of GvHD/relapse free survival (GRFS), for the entire population the 4-year GRFS was 17,8%.
Day-100 cumulative incidence of grade II-IV and III-IV acute GvHD were 30,1% and 15,5% respectively.
The 4-year cumulative incidence of chronic GvHD was 33,5% (n 74) - moderate-severe chronic GvHD 26,2%.
According to the original CIBMTR RG the 3-year OS in each RG was RG1 (n 1) 100%, RG2 (n 42) 83%, RG3 (n 26) 34% and RG4 (n 5) 20% (p < .0001).
According to the revised CIBMTR RG the 3-year OS in each rRG was rRG1 (n 1) 100%, rRG2 (n 31) 78%, rRG3 (n 32) 62% and rRG4 (n 10) 10% (p < .0001) with a 3-year NRM of 0% - 10% - 15% - 43% respectively (p .002).
Conclusions
Overall, irrespective of donor, cGvHD affects 20-50% of HCT, it is the leading cause of death in pts who survive at least 2 years without relapse, it is associated with worse quality of life and functional status. Of note, 30% of pts diagnosed with cGvHD 5 year later are still on treatment and 30% have relapse or died. Considering this context, the refined CIBMTR risk score provides a valid support in the prognostic stratification of pts at onset of cGvHD also after haplo-HCT.
Marktel: GSK: Other: B-thalassemia gene therapy was developed by Fondazione Telethon and Ospedale San Raffaele and has been inlicenced by GSK that provides funding for the clinical trial, Research Funding. Ciceri: GSK: Other: B-thalassemia gene therapy was developed by Fondazione Telethon and Ospedale San Raffaele and has been inlicenced by GSK that provides funding for the clinical trial, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal